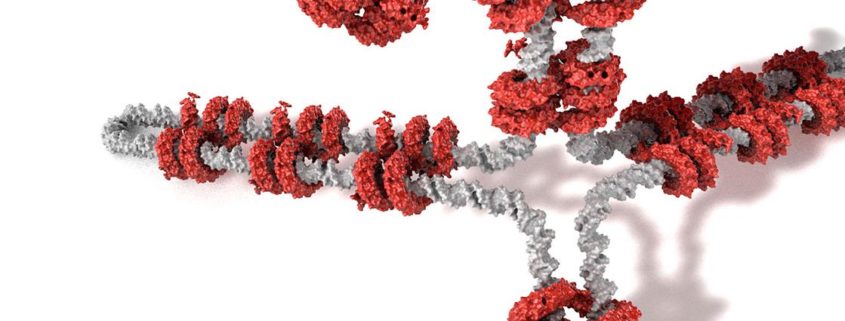
Europe says University of California deserves broad patent for CRISPR
Nine months since the British vote to exit the European Union (“Brexit”), the UK science community’s initial dismay has given way to hard-boiled determination to limit the damage it will do to universities and research. On 29 March, Prime Minister Theresa May is expected to give formal notification of the UK’s intention to withdraw under Article 50 of the Lisbon Treaty, the constitutional basis of the EU. This will set in motion a 2-year period of intense negotiation on the terms of the UK’s divorce, and any future agreements with the EU—with research just one line item on a long list of issues to be resolved.